In part one we talked about Buffer selection & it’s correlation in terms of solubility, pH & buffer capacity range along with the Buffer concentration & an important term which could affect the baseline in the method development, this term is called UV Cut-off the Method development, analyte detection & quantification, In this part our main focus would be about the effect of Buffer selection on the Analyte’s retention, peak shape, resolution & selectivity on the HPLC column. We would also address here the impact of a varying injection volume on the Buffer capacity & so on, the Analyte’s peak shape.
Buffer selection -Impact of pH on method development –(Analyte Retention)
pH is a very crucial factor in HPLC method development of ionizable compounds, since their retention is very sensitive when it comes to the mobile phase pH. As mentioned in the 1st part, the buffer aims to resist any change in pH upon the addition of minor amounts of an acid or base. Many different substances have been used for buffering in HPLC We would mention the most important additives & salts used for buffer preparation as follows:
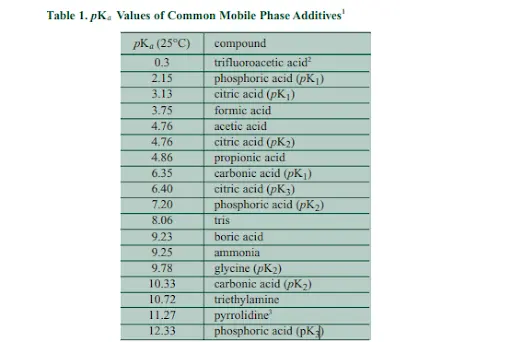
As a general note in method development, it is better to start with pH 2-3 range since at such pH, the majority of organic acids ionization would be suppressed-meaning less polar ie more hydrophobic- which indicates a stronger retention on the reversed phase on HPLC column which is the main aim for our method development, also the ionization of the silanol groups on the column would be suppressed which is also very favorable in method development & this would be explained later on.
To further illustrate, for a molecule to retain on a reversed phase HPLC column, it needs to be more hydrophobic, this is guaranteed in case of a non-ionizable molecule so for an acid molecule to remain non-ionizable, we need to prepare a mobile phase pH that is 2 units below the analytes pKa, in such case the molecule would be >99% unionized & vice versa for a bases where in such case we need to prepare a mobile phase pH that is higher 2 units than the analyte’s pKa, so to summarize acids would be more retained at low pH(figure 1a) while for bases they would be more retained at high pH (figure 1b)
Impact of pH on method development –(Analyte separation & selectivity on HPLC column)
The more the mobile phase pH is near the analyte’s pKa, the more it would be obvious for a small change in pH to give a significant change in retention, which is unfavored for method development since it indicates the lack of a robust separation. Below in figure 2a, you can see the impact of any minor change in pH even if it was as small as just 0.1 units such as in this case-knowing that this amount of error is very common in many laboratories in pH adjustment, yet you can see how even such a minor change in pH made a huge difference which was impacted on retention & thus resolution as well. At pH 5, retention is less sensitive to pH than it is at pH 3 in the case of the acid molecule or pH≥6 for the base.
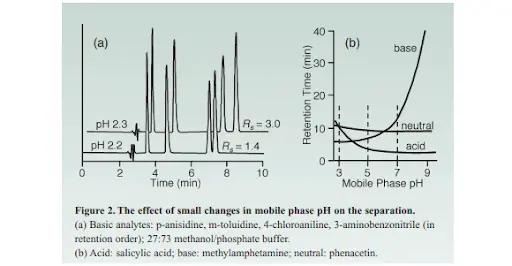
From the above figure ,It is obvious that not only retention time is affected when the pH is near the pKa ,but also selectivity could change in case of molecules of similar structure were found.
HPLC column stability & type of Silica
As mentioned earlier in the 1st part of this topic, most of the HPLC columns are stable in the pH range of 2-8. Below pH 2, hydrolysis could occur, causing bonded phase loss, while above pH 8, the silica backbone. Adding to the previous, the major cause for basic compounds peak tailing has been their interaction with the silanol groups that have been ionized by a cation exchange process yet nowadays the newly used Type B Silica is of high purity with a pKa of >7 which eliminates the factor of silanol sites ionization to a great extent, that’s the reason behind why the high purity silica gives a way high better peak shape for bases than the older type of silica, In the below graph you can see how the usage of a high purity silica in the HPLC column has improved the peak tailing for bases to a great extent.
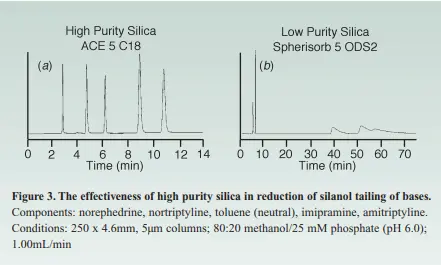
Moreover, an improvement of reproducibility is seen upon the usage of a high-purity silica compared to a low-purity one because of the reduction of such unpredictable secondary silanol groups. As a practical implication to summarize the above-mentioned data.
So to summarize all of the above, it is generally recommended based on the characteristics of the column& the samples in general to start the development of the method with a mobile phase pH within pH 2-3, normally at this pH suppression for the ionization of organic acids would occur so does the silanol groups on the column ionization as well yet at such pH Bases would be ionized, suppressing for the ionization for such compounds of usually having pKa>7 would require operating at higher pH which usually would case deterioration of most of HPLC columns. If operating at high pH is crucial, then be sure to choose a durable HPLC column at such pH.
Generally, adjusting the organic content of the mobile phase(%B-solvent) is usually more fruitful at first to begin with regarding obtaining an acceptable retention for either neutral &non-ionized compounds, then to ensure pH adjustment to obtain suitable retention for the ionic analytes.
Correlation of Sample Injection Volume with Buffer
1. Purpose of Using Buffer in HPLC
- The main goal of a buffer is to maintain the analyte at the required pH.
- Only a minimal amount of buffer is typically needed because sample concentrations are usually in the µg/ml to ng/ml range.
- When the injection volume is < 100 µl, the mass on the column is typically just a few hundred nanograms.
2. Buffer Interaction with HPLC Column
- The buffer must maintain a constant pH on the HPLC column.
- A large volume of buffer passes through the column during analysis, constantly exposing the silica.
- Modern HPLC columns contain high purity silica with fewer acidic silanol groups, requiring less buffer compared to older, lower purity silica columns.
3. Effect of TFA Concentration on Peak Shape
- Trifluoroacetic acid (TFA) serves two roles:
- Maintains low pH
- Acts as an ion-pairing reagent
- These effects are interrelated, not isolated.
4. Chromatogram Observations
- Comparing 0.1% vs. 0.01% TFA, there’s a noticeable difference in peak shape.
- The purity of the silica also affects the degree of peak tailing:
- Lower purity silica shows more degradation in peak shape at lower TFA concentrations.
- Moderate and low-purity columns are especially sensitive.
5. Conclusion from Observations
- TFA significantly buffers the column and prevents secondary interactions between the analyte and the column.
- Such unwanted interactions cause peak tailing.
- Insufficient buffer impacts peak shape regardless of silica purity.
6. Buffer Concentration Recommendations
- For acid additives (like TFA):
➤ Recommended concentration = ~0.1% v/v - For buffer solutions:
➤ Recommended molarity = 5–10 mM
7. Sample Volume vs. Buffering Efficiency
- The primary role of the buffer is to adjust the sample pH to match the mobile phase.
- Smaller sample volumes allow the buffer to act more effectively.
- With larger sample volumes, pH deviation can occur, weakening the buffer’s effect.
- You can reduce the need for high molarity buffers by adjusting the sample pH before injection.
References
The LCGC Blog: Buffer Choice for HPLC Separations (chromatographyonline.com)